#InCodeWeTrust
WHAT WE DO?
THEY RELY ON US
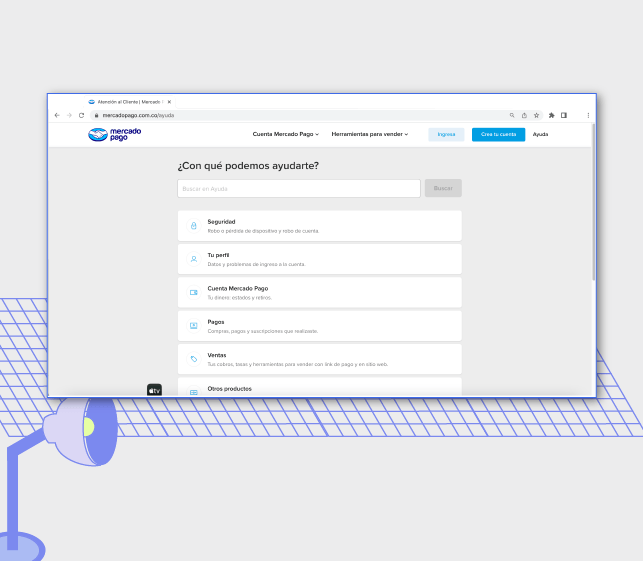
MercadoPago Integration
"Bitlogic quickly adjusted to Mercado Libre's work pace. They shared their insights, added a personal touch, and kept communication smooth." - Tomas Palaia, Software Manager, Mercado Libre.
Being strategic partners
"We like Bitlogic for its teamwork in reaching goals. We appreciate the quality of their work and their technical skills. We also value the strong relationship they build with clients." Gustavo Burgi, CTO, Online Education Center.
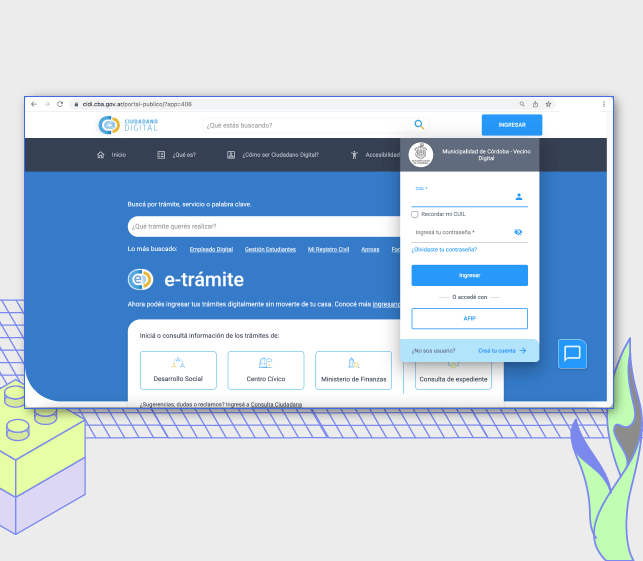
City Appointment System
"Modernizing a public entity takes a lot of work. Most importantly, it needs a strong understanding of how things operate to change the processes." - Cr. Ariel Marcelo Vercellone, Director of Public Improvement, City of Córdoba.
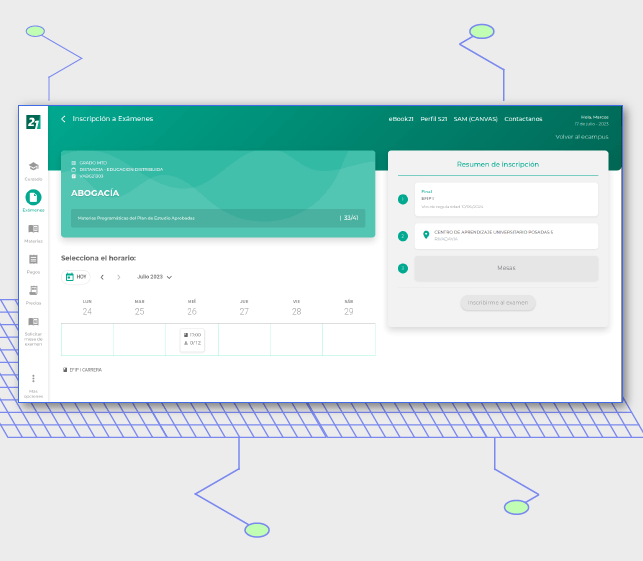
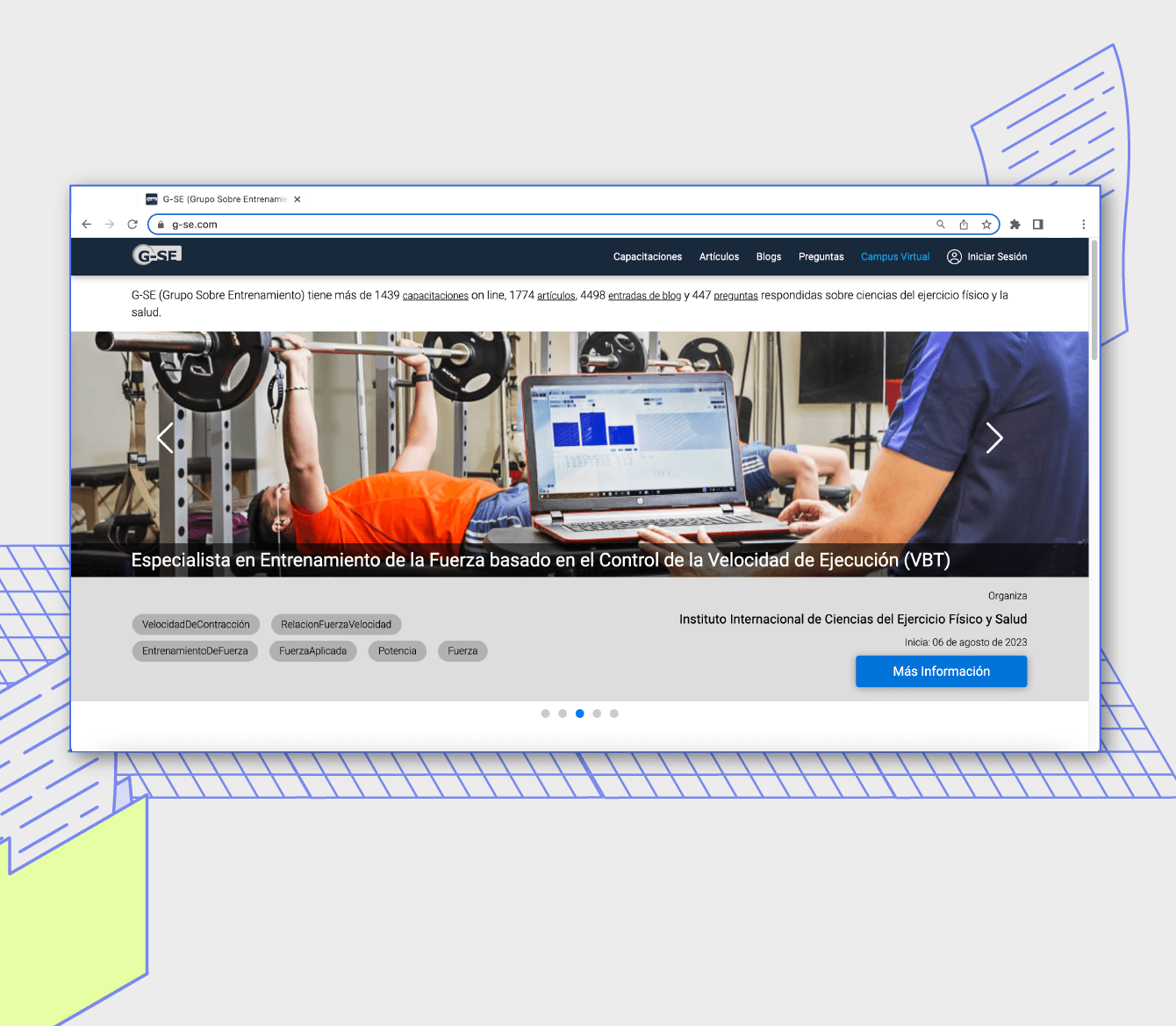
New experience for students
"Bitlogic provided us with a profound digital transformation that enables us to propel ourselves into the future of education." Hugo Colombatto, IT Director, University Siglo 21.
Enhancing the learning experience
"With Bitlogic, we found availability in addressing our inquiries and constant support." Yamil Alejandro Rabbat, Co-Founder & CEO, Ed Machina.
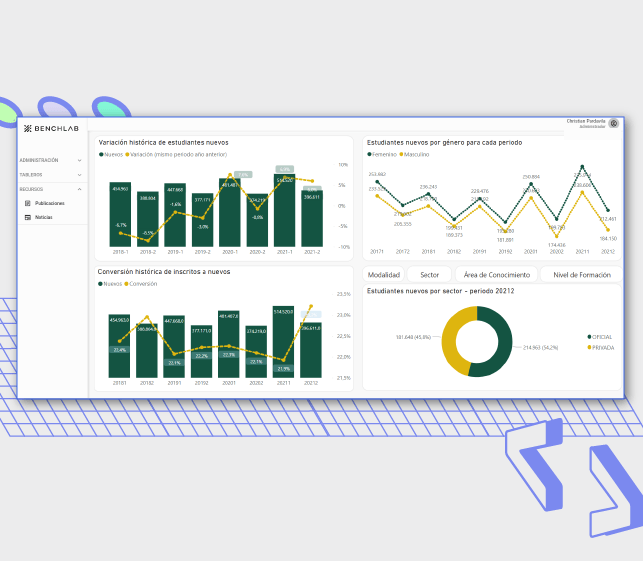
Self-management and Scalability
“Bitlogic provided us with high-quality user experience and allowed us to scale while ensuring security and performance." Jorge Eduardo Silvestre, Product and Operations Manager, Benchlab.



















































"At Bitlogic, you learn something new every day; it's great to be surrounded by experts."
Jesi, Solution Architect